Hydrodynamic influences on
biofilm formation and growth
Alison Kraigsley
and Paul D. Ronney
Department of Aerospace and Mechanical
Engineering
University
of Southern California, Los Angeles, CA 90089-1453
Steven
E. Finkel
Department of Biological
Sciences
University of Southern
California, Los Angeles, CA 90089-1340
Not proficient in English?
Abstract
Biofilm
formation is a major factor in the growth and transport of both desirable and
undesirable bacteria as well as fouling and corrosion. While much is
known about self-propagating reaction-diffusion fronts that occur in many
chemically reacting systems such as flames, polymerization processes and some
aqueous reactions, this vast knowledge base has not previously been
systematically applied to biological systems such as motile bacteria or the
spread and growth of biofilms. We have initiated a systematic of the study the influences
of hydrodynamics on biofilm formation and growth, using a simple flow tube
apparatus using Escherichia coli.
Biofilm formation was monitored using a modified Gram-stain protocol and
quantitative spectrophotometric assays. Initial experiments do indeed show
behavior analogous to reaction-diffusion systems.
Introduction
Biofilms
are complex communities of surface-attached microorganisms, comprised either of
a single or multiple species (Costerton, 1995; Davey
& O’Toole, 2000). Over the past
few decades, there has been a growing realization that bacteria in most
environments are not found in a unicellular, planktonic (free-living) form such
as those typically studied in the laboratory, but exist predominantly in
multi-cellular surface attached communities called biofilms (Costerton et al., 1995). This realization has spurred much
research into the physical and chemical properties of biofilms, the
characterization of their morphology, and the mechanisms of their development.
Biofilms
are found ubiquitously in virtually all natural,
medical, and industrial settings where bacteria exist (Costerton,
1995; Costerton et al., 1995; Davey and O’Toole, 2000).
Biofilms can form in almost any hydrated environment that has the proper
nutrient conditions, and can develop on a wide variety of abiotic hydrophobic
and hydrophilic surfaces, including glass, metals, and plastics (Miller &
Ahearn, 1987; Marshall, 1992; Fletcher,1998; O’Toole
& Kolter, 1998ab). Biofilms also readily form on biotic
surfaces including human skin and epithelial cells. Generally surface material does not
strongly affect biofilm growth.
Examples of bacterial biofilms are chronic P. aeruginosa
infections in the lungs of cystic fibrosis patients, oral microbes on teeth, the "slime" layer on the surface of submerged
objects in aquatic environments, biofouling of water
supply, sewage, and oil pipelines, and bacterial colonization of plant surfaces. The transition from the planktonic,
free-swimming, mode of existence to a biofilm is a regulated developmental
process that leads to a complex surface-attached bacterial community (O’Toole
et al., 2000). This biofilm
community has a number of distinct characteristics including the production of exopolysaccharides, the formation of chemical and pH
gradients, a marked degree of structural heterogeneity, and the development of
high level resistance to a wide variety of biocides (Hoyle et al, 1992). Formation of biofilms can have profound
negative and positive impact in these environments, and, as a consequence, can
have high costs in terms of both economics and human health.
Mechanism
and genetics of biofilm formation
A
bacterial biofilm begins to form when individual cells initially attach to a
surface (Costerton, 1995; O’Toole & Kolter, 1998b).
The ability of a cell to perform this “initial attachment event” is
controlled by both environmental factors, including nutrient levels,
temperature, and pH, and genetic factors, including the presence of genes
encoding motility functions, environmental sensors, adhesins,
etc. (Costerton, 1995; O’Toole et al., 2000). The combination of factors influencing
biofilm development are frequently species-specific, however, there are many
features common to most bacteria studied to date. After initial attachment, the cells
begin to grow and spread as a monolayer on the surface to form microcolonies.
During microcolony formation, cells undergo
developmental changes which give rise to the complex
architecture of the mature biofilm.
Paramount among these changes are the
production of the exopolysaccharide (EPS) matrix, one
of the hallmarks of a mature biofilm (Costerton,
1995; Danese et al., 2000). As the biofilm continues to grow several
things can happen; the biofilm may spread into uninfected areas as
environmental conditions allow and, occasionally, cells will detach from the
biofilm and re-enter a planktonic mode.
These planktonic cells can then repeat the cycle, infecting new
surfaces.
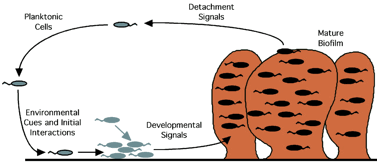
Figure 1. Schematic diagram of biofilm formation
and growth (after O’Toole et al.,
2000)
As
stated, bacteria will form biofilms on many biotic surfaces and virtually all
abiotic surfaces. The ability to attach
to a wide variety of plastics, glass, and metals is mediated by specific
surface proteins and appendages (O’Toole & Kolter, 1998a; Pratt &
Kolter, 1998). Also important for
initial attachment is the ability of bacteria to “swim” using flagella for propulsion,
referred to as flagellar-mediated motility. Non-swimming bacteria have reduced
biofilm forming ability. For most
Gram-negative motile bacteria, approximately 1% of the genome is devoted to
flagellar function. Another form of
bacterial motility, referred to as “twitching” motility, is not mediated by the
rotation of flagella, but is due to the extension and retraction of another
appendage called pili (O’Toole & Kolter, 1998a). Unlike flagellar-mediated swimming,
twitching motility occurs only when cells are attached to a surface and the
bacteria slide themselves across that surface. Twitching is important for both
the formation of microcolonies and spreading of biofilm communities.
Medical
and industrial costs of biofilms
Bacterial biofilms cause “biofouling”
in a wide variety of industrial settings.
Biofilms grow inside pipelines transporting a myriad of substances,
including potable water, oil, chemicals and fire extinguishing agents (Fig. 2). Costs associated with biofilm
contamination are due to both constriction of pipeline diameter, reducing
transmission rates, and due to contamination. In marine settings, biofilms reduce the
hydrodynamic efficiency of ships and propellers. Fire
protection systems represent a particularly complex challenge for biological
fouling prevention and control (Mittelman, 2001). Fluid flow is nearly always stagnant,
and the piping conduits are not designed to facilitate routine cleaning
operations. Pitting corrosion occurring under deposits in fire protection
systems can be initiated or propagated by these microbial activities. In
pipeline applications, through-wall penetration of carbon steel and copper has
been reported within months after a new line has been brought into service. This can cause occlusion of pipelines,
sometimes completely blocking flow in six-inch diameter pipelines. The costs of disinfection,
cleaning and replacement of biofilm-contaminated material run into the hundreds
of billions of dollars per year worldwide.
It has been shown that biofilm grown
cells can become 10-1000X more resistant to the effects of antimicrobial agents
than their planktonic counterparts (Brown et al., 1988; Hoyle and Costerton,
1991; Ashby et al., 1994; Costerton et al., 1995; Koenig et al, 1995; Stewart,
1996; Lewis, 2001; Mah & O’Toole, 2001). Biofilms show resistance to a wide range
of antibiotics (including ampicillins, strepotomycin, tetracyclines,
gentamicin, and many others) and biocide oxidants such as ozone, chlorine and
iodine. This characteristic of biofilms
makes them extremely difficult to control in both medical and industrial
settings. Traditional antibiotic
therapy can eliminate sensitive planktonic bacteria, but these same organisms
when growing in a biofilm can survive treatment. For example, when biofilms grow on the
surfaces of medical implants requiring antibiotic treatment, the therapeutic
levels of antibiotic required to eliminate biofilm bacteria often cannot be
achieved in the patient or are toxic (Barie et al., 1990). Therefore, biofilm-based infections can
become chronic with the only recourse being removal of the contaminated
implant. Biofilm-associated
infections extend hospital stays an average of about three days and it is
estimated that up to 65% of nosocomial infections are biofilm-based with an
associated treatment cost in excess of $1 billion per year. Biofilms formed on indwelling medical
devices (Fig. 3) serve as a reservoir of bacteria that can be shed into the
body, leading to a chronic systemic infection. Indeed, up to 82% of nosocomial
bacteremias are the result of bacterial contamination of intravascular
catheterizations (Archibald & Gaynes, 1997). Other examples of medically
significant biofilms include oral microbes on teeth, chronic Pseudomonas
aeruginosa infections in the lungs of cystic fibrosis patients and
bacterial contaminants on medical devices such as pacemakers and catheters.
|
|
|
|
|
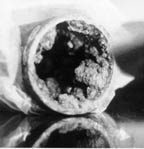
Figure
2. Left: Tubercle formation in a
carbon steel fire protection pipe. Iron oxidizing bacteria were found in
association with the tubercles.
Right: Bacterial biofilm associated with stainless steel tube. Scanning electron micrograph
magnification at 5,000X (Mittelman, 2001). |
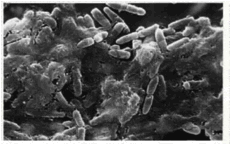
Figure 3. Electron micrograph of interior
surface of a vascular catheter removed from a patient showing growth of a
bacterial biofilm of P. aeruginosa. (Yassien et al., 1995). Biofilm bacteria leaving the catheter
can cause sepsis. |
|
Dynamics
of biofilm formation
It is known that motile bacteria form biofilms more readily
than cells that cannot perform flagellar-mediated swimming, but the reasons for
this requirement are not fully understood (Fletcher, 1988; O’Toole &
Kolter, 1998a; Pratt & Kolter, 1998).
Cells that do not exhibit twitching motility, because they lack the pili
genes, still form biofilms, but they do not achieve the characteristic biofilm
architecture of wild-type cells.
Hyperpiliated mutants, which also do not twitch, adhere to surfaces even
better than wild-type cells, but also show altered morphologies (Gibbs &
O’Toole, manuscript in preparation).
The study of the formation of biofilms in terms of cell motility have
generally focused on the ability of the cell to locomote; less attention has
been focused on characterizing the effects of the flow of the liquid
environment on biofilm formation.
There is increasing interest in these questions and many investigators
are now using various forms of flow cell technology to characterize biofilm
formation. However, many of these
studies have been focusing either on the kinetics of early attachment events or
characterizing the morphologies of biofilms grown under differing flow regimes. What is lacking is a systematic study of
the effects of hydrodynamics (e.g. flow rates and shear forces) on the
formation, spread, and persistence of biofilms. Studies characterizing hydrodynamics
effects on biofilm formation can address many fundamental questions. For example, cells adhere to water on a
surface, how do they gain a foothold?
In a deep layer of water, motile cells adhere to the surface, but
non-motile cells do not. In order
for cells to colonize a surface and form a biofilm, they need to reach the
surface. Is motility required for
biofilm spread as opposed to growth and maturation at a fixed location?
While a number of investigations of the
flow characteristics on biofilm formation (e.g. Heydorn et al., 2000)
have been performed, these studies only report the volumetric flow rate (or
sometimes mean flow velocity (um), i.e. the velocity averaged over
the cross-section of the flow channel).
The flow environments are not well characterized in terms of flow
velocity profiles at the biofilm growth location. Key questions have not been addressed,
e.g. is mean flow velocity (um) sufficient or relevant to
characterize biofilm growth? Due to
the hydrodynamic no-slip condition, the flow velocity at the surface where
the biofilm is growing is always zero – does this mean that the
biofilm can attach and grow no matter how strong the flow? This seems unlikely. In order to predict momentum, heat and
mass transfer, it is well known in the fluid mechanics literature that the gradient
of velocity, temperature and composition at the surface, not the mean
value of velocity, temperature or composition itself is the key factor
affecting transport since all of these are gradient-transport properties (i.e.
if the velocity, temperature or composition are uniform there is no flux of
momentum, heat or mass, respectively).
As an example of these effects,
consider motile planktonic Escherichia coli attempting to colonize a
surface and form a biofilm. E.
coli swim at typically 20 Ķm/s (Berg, 2000) and are about 1 Ķm in diameter,
and thus can produce a fluid velocity gradient of about 20 Ķm/s / 1 Ķm =
20/s. If the local velocity
gradient at the surface (in particular the shear rate ∂u/∂y, i.e. the gradient
of velocity (u) in the direction (y) perpendicular to the velocity) is
significantly smaller than this value, the E. coli can swim to the
surface (where u = 0) and remain within a distance from the surface equal to
their size without being dispersed.
For laminar flow inside a cylindrical tube, the velocity gradient ∂u/∂r
(i.e. the radial gradient of axial velocity) at the wall is 8um/d,
where d is the inside diameter of the tube. For a flow rate of 1 ml/min in a tube of
1/8” inside diameter, this corresponds to um = 0.21 cm/s and ∂u/∂r =
5.3/s, which should be well within the swimming capability of the
bacterium. If the flow rate were increased
to 3.8 ml/min, ∂u/∂r would be 20/s and the E. coli might have much more
difficulty colonizing the surface because the side of their body away from the
wall would experience a fluid velocity equal to its swimming speed capability,
and so would wind up tumbling along the wall rather than adhering to it –
despite the fact that the fluid velocity at the wall is zero.
One reason for the lack of
characterization and quantitative prediction of fluid flow and motility effects
on biofilm formation and growth is the absence of an appropriate modeling
foundation. The number of organisms
in a macroscopic biofilm is far too large to track each individual. It is standard in many fields (e.g.
chemistry, nuclear physics, macroeconomics) to use thermodynamically-based
models in which the behavior of ensemble averages rather than individuals (e.g.
molecules, sub-atomic particles, consumers) is analyzed to predict system
performance. A natural choice for a
thermodynamic approach to studying biofilm formation is the reaction-diffusion
system because the biofilm grows and/or spreads in response to the
transport (via diffusion and convection) of “reactants” (nutrients) to the
“products” (individual bacterium) that then generate more products and cause
the population of products to spread.
(The term “reaction-diffusion” is generally understood to encompass
convective as well as diffusive transport where appropriate). While much is known about
reaction-diffusion systems that produce self-propagating fronts in many
chemically reacting systems such as flames, polymerization processes and some
aqueous reactions, this vast knowledge base has not previously been
systematically applied to microbiological systems such as motile bacteria or
spreading biofilms. For the use of
reaction-diffusion models to be valid it is necessary to demonstrate, at least
in concept, that reaction-diffusion behavior is exhibited in biofilms. This led us to perform the feasibility
tests described below. The results
do show that these hypotheses are in fact valid in principle.
Feasibility tests
Laminar flow in a cylindrical tube is a
simple and well-characterized flow system, and so was chosen for our initial
tests of flow effects on biofilm formation. Figure 4 shows the experimental
apparatus and procedure we used. 10
cm long pieces of transparent Tygon PVC tubing of 1/8” inside diameter were
placed for 12 hr at 37ŻC in a medium of Luria-Bertani (LB) nutrient broth + E.
coli with half of the tube immersed in the medium and half exposed to
air. This incubation period results
in the formation of a dense biofilm near the air-medium interface due to the
abundant supply of oxygen there. As
seen in Fig. 5 (upper left, “control” case), a biofilm of much lower density
forms in the submerged region and of course no growth occurs far above the
interface. These samples were then
placed in the flow system composed of an LB reservoir, peristaltic pump,
pluming connections for the test samples, and a waste tank for LB after it
passes through the sample tube.
Both flow rate and test duration were varied.
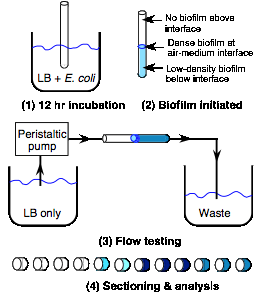
Figure 4. Schematic diagram of apparatus and
procedure for testing of flow effects on biofilm formation
Following O’Toole (1999), quantification of attachment of
biofilm bacteria was accomplished by staining cells with the dye Crystal Violet
(CV). CV will specifically stain
bacteria (minimal staining of tubing material has been observed.) After staining and extensive washing, CV
is solubilized by treatment with methanol and quantitated by determining the
absorbance at 550 nm (A550) in a spectrophotometer. Experiments (O’Toole, 1999)
have shown a quantitative relationship between the A550 measured and the number
of colony forming units (CFU) of biofilm bacteria.
Figure 5 shows typical images of CV-stained biofilms
resulting from these tests. It can
be seen that for a fixed flow rate (left side, lower 3 images), the density of
the film increases over time as expected.
It is interesting to note that the biofilm can spread upstream of
the location of the initial biofilm with a speed (for times < 12 hr) on the
order of 0.5 mm/hr. This upstream
spread is analogous to a flame spreading over a solid fuel bed (e.g. wood,
paper, plastic) in the upwind direction.
This phenomenon has been widely studied (e.g. de Ris (1969),
Fernandez-Pello et al. (1980), Honda and Ronney (1998)) in the fire
safety community and it is well known that under many circumstances the upwind
spread is increases as the wind speed increases due to the increased
rate of oxygen transport to the flame.
It is also interesting to note that the spread seems to show a marked
change between 12 and 24 hr, where a low-density film appears in the upstream
region. Figure 5 (right column)
shows the effect of flow rate for a fixed test time (3.5 hr). It can be seen that there is an
optimal flow rate on the order of a few ml/min that maximizes both the upstream
(of the initial dense region) progress of the biofilm and the density of the
downstream growth. This is
consistent with the suggestion that a flow velocity exceeding 3.8 ml/min (for a
1/8” I.D. tube), which corresponds to a shear rate ∂u/∂r at the wall of 20/s,
is the maximum shear that the E. coli can readily withstand without dispersing. It is also worth nothing that at high
shear rates (Fig. 5, lower right), the biofilm growth becomes very non-uniform
and forms patches rather than coating the surface contiguously. This behavior is often found in
reaction-diffusion systems, i.e. that at small shear rates, an increase
in shear rate increases the flux of “reactants” (LB nutrients in this case) to
the “reaction zone’ (the biofilm surface), thus increasing the rate of
reaction, whereas too high a shear rate discourages reaction (because of
insufficient residence time which results in loss of “heat” (bacterium in this
case) from the reaction zone.)
Moreover, at high shear rates the fire spreading process becomes
unstable and leads to non-uniform spread (e.g. Wichman, 1999, Wichman and
Olsen, 1999).
It should be noted that even for the highest flow studied
(16 ml/min), the Reynolds number (Re) = umd/n, where n is the
kinematic viscosity, is only 107, which is well below that required for
transition to turbulent flow (Re Ň 2000), so turbulence effects cannot explain
the patchy nature of the biofilms at the high flow rates.
Additional tests (not shown) in which the medium flowing
through the sample tubes included planktonic E. coli as well as LB,
generally showed less biofilm formation for the same flow rate and test
duration, presumably because the planktonic bacteria were competing with the
biofilm for nutrients. It might
have been expected that the presence of planktonic bacteria might increase
biofilm growth due to “recruitment” of planktonic bacteria by the biofilm, but
there was no evidence to support this suggestion. Thus, we conclude that generally the
biofilms we observed grew by spreading along the surface rather than by
recruitment from the flowing media – recruitment is neither necessary nor
desirable. This again supports the
notion of modeling biofilms as reaction-diffusion systems.
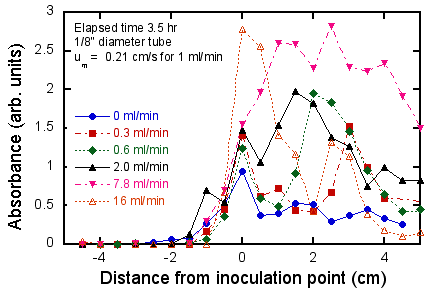
These qualitative findings are supported by the quantitative
data based on CV-A550 measurements (Fig. 6). In Fig. 6 (upper), the effect of time
spent in the flow cell on the increase in biofilm density, as well as the
formation of the biofilm far upstream of the initial biofilm inoculation
region, is clearly seen. It can
also be seen that (at least for a flow rate of 0.6 ml/min) in the downstream
region the growth “saturates” after about 3.5 hr and after that time the growth
is primarily spreading upstream.
There is no evidence of “attrition” from the biofilm in that the biofilm
density does not decrease at later times, at least up to 24 hr. In Fig. 6 (lower), it can be seen that
the effect of flow rate is non-monotonic, namely that the densest growth occurs
at an intermediate flow rate, and that for higher or lower flow rates the
growth rate decreases substantially.
In particular, between 7.8 ml/min (∂u/∂r at the wall = 42/s) and 16
ml/min (∂u/∂r = 85/s) the growth downstream of the inoculation point drops by a
factor of at least 2. This range of
∂u/∂r is comparable to the predicted transition value of 20/s expected for E.
coli. Also, the patchy nature
of the growth at high flow rates can be seen in the fluctuations in absorbance
as a function of distance. Finally,
it can be see that as the flow rate increases, the steepness of the biofilm
front (i.e. the slope of the plot of biofilm density vs. distance) increases
also. This is consistent with the
notion of a convection-diffusion zone at the upstream edge of the biofilm whose
thickness scales as (D/(∂u/∂r))1/2 (Williams, 1985), i.e. the
steepness increases as the flow rate (and thus stretch rate) increases.
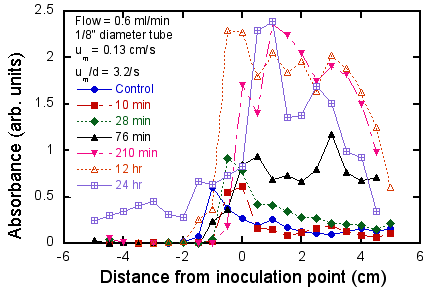
Figure 6. Quantitative measurements of effect of
flow and test duration on biofilm growth.
Upper: fixed flow rate (0.6
ml/min), varying test duration; lower: fixed test duration (3.5 hr), varying
flow rate. Negative distances refer
to regions upstream of the inoculation point (i.e. the location of the medium-air
interface where the dense biofilm was pre-formed (see Fig. 4).
References
Archibald, L. K., and R. P.
Gaynes. 1997. Hospital
acquired infections in the United States: the importance of interhospital
comparisons. Nosocomial Inf. 11(2):245-255.
Ashby, M. J., J. E. Neale, S.
J. Knott, and I. A. Critchley.
1994. Effect of antibiotics on non-growing planktonic cells and biofilms of
Escherichia coli. J Antimicrob Chemother. 33(3):443-52.
Ausubel, F. A., R. Brent, R.
E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1990. Current Protocols in Molecular
Biology. Wiley Interscience, New York.
Barie, P. S., N. V. Christou,
E. P. Dellinger, W. R. Rout, H. H. Stone, and J. P. Waymack. 1990. Pathogenicity of the
enterococcus in surgical infections. Ann. Surg. 212(2):155-158.
Berg, H. C., "Motile Behavior of
Bacteria" Phys. Today 53, 24 (2000).
Bloemberg, G. V., G. A. O'Toole, B. J. J. Lugtenberg, and R. Kolter. 1997. Green fluorescent protein as a marker for Pseudomonas spp. Appl. Environ. Microbiol. 63(11):4543-4551.
Brooun, A., S. Liu, and K.
Lewis. 2000. A
dose-response study of antibiotic resistance in Pseudomonas aeruginosa
biofilms. Antimicrob Agents Chemother. 44(3):640-6.
Brown, M. R., D. G. Allison,
and P. Gilbert. 1988.
Resistance of bacterial biofilms to antibiotics: a growth-rate related effect?
J Antimicrob Chemother. 22(6):777-80.
Budrene E.O., Berg H. C., "Complex patterns formed by
motile cells of E. coli," Nature 349, 630 (1991).
Cochran, W. L., G. A.
McFeters, and P. S. Stewart.
2000. Reduced susceptibility of thin Pseudomonas aeruginosa biofilms to
hydrogen peroxide and monochloramine. J Appl Microbiol. 88(1):22-30.
Cormack, B. P., R. H.
Valdivia, and S. Falkow. 1996. FACS-optimized mutants of the
green fluorescent protein (GFP). Gene. 173:33-8.
Costerton,
J. W. 1995. Overview of
microbial biofilms. J. Indus. Microbiol. 15:137-140.
Costerton, J. W., Z.
Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms, p. 711-745. In
L. N. Ornston, A. Balows, and E. P. Greenberg (ed.), Annu. Rev. Microbiol.,
vol. 49. Annual Reviews, Inc., Palo Alto, CA.
Danese, P. N., L. A. Pratt,
and R. Kolter. 2000.
Exopolysaccharide production is required for development of Escherichia coli
K-12 biofilm architecture. J Bacteriol. 182(12):3593-6.
Datsenko K. A. and, B. L.
Wanner. 2000. One-step
inactivation of chromosomal genes in Escherichia coli K-12 using PCR
products. Proc Natl Acad Sci U S A. 97:6640-5.
Davey, M. E., and G. O.
O'Toole. 2000. Microbial
biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Revs. 64(4):847-67.
De Kievit, T. R., M. D.
Parkins, R. J. Gillis, R. Srikumar, H. Ceri, K. Poole, B. H. Iglewski, and D.
G. Storey. 2001. Multidrug
efflux pumps: Expression patterns and contribution to antibiotic resistance in Pseudomonas
aeruginosa biofilms. Antimicrob Agents Chemother. 45(6):1761-70.
deRis, J. N. 1969. Spread of a laminar diffusion
flame. Twelfth Symposium
(International) on Combustion, The Combustion Institute, Pittsburgh, 1969,
p. 241.
Epstein, I. R. Pojman, J. A. 1998. An introduction to nonlinear
chemical dynamics, Oxford.
Fernandez-Pello, A. C., Ray,
S.R., Glassman, I. 1981. Eighteenth Symposium (International)
on Combustion, The Combustion Institute, Pittsburgh, p. 579.
Fletcher, M. 1988. Attachment of Pseudomonas
fluorescens to glass and influence of electrolytes on bacterium-substratum
separation distance. J. Bacteriol. 170(5):2027-2030.
Govan, J. R. W., and V.
Deretic. 1996. Microbial
pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and
Burkholderia cepacia. Microbiol. Rev. 60(3):539-574.
Heydorn, A., B. K. Ersboll, M. Hentzer,
M. R. Parsek, M. Givskov, and S. Molin.
2000. Experimental reproducibility in flow-chamber biofilms. Microbiology. 146(Pt
10):2409-15.
Heydorn, A., A. T. Nielsen, M.
Hentzer, C. Sternberg, M. Givskov, B. K. Ersboll, and S. Molin. 2000. Quantification of biofilm
structures by the novel computer program COMSTAT. Microbiology. 146(Pt
10):2395-407.
Honda, L. K. and Ronney, P. D.
1998. "Effects of Ambient Atmosphere
on Flame Spread at Microgravity,” Combustion Science and Technology
133:267-291.
Hoyle, B. D., and W. J.
Costerton. 1991. Bacterial
resistance to antibiotics: the role of biofilms. Progress in Drug Research. 37:91-105.
Koenig D.W., S. K. Mishra, and
D. L. Pierson. 1995. Removal of
Burkholderia cepacia biofilms with oxidants. Biofouling. 9:51-62.
Koenig, D.W., and D. L.
Pierson. 1997.
Microbiology of the Space Shuttle water system. Water Sci Technol. 35:59-64.
Kuchma, S. L., and G. A.
O'Toole. 2000.
Surface-induced and biofilm-induced changes in gene expression. Curr. Opin.
Biotech. 11:429-433.
Lewis, K. 2001. Riddle of biofilm resistance.
Antimicrob Agents Chemother. 45(4):999-1007.
Lewis, B., von Elbe, G., Combustion, Flames, and Explosions of Gases, 3rd
ed., Academic Press, 1987.
Mah, T.-F., and G. A. O'Toole. 2001. Mechanisms of biofilm resistance
to antimicrobial agents. TIMS. 9:34-39.
Marshall, K. C. 1992. Biofilms:an overview of
bacterial adhesion, activity, and control at surfaces. ASM News. 58(4):202-207.
Maruta, K., Takeda, K., Ahn,
J., Borer, K., Sitzki, L, Ronney, P. D., Deutchman, O. (2002). Extinction Limits of Catalytic
Combustion in Microchannels, To appear in the Proceedings of the Combustion
Institute, Vol. 29.
McLean, R.J.C., Cassanto,
J.M., Barnes, M.B., Koo, J.H., 2001. Bacterial biofilm formation under
microgravity conditions. FEMS
Microbiology Letters 195, 115-119.
Miller, J.H. 1992. A short course in bacterial
genetics. Cold Spring Harbor
Laboratory Press, Cold Spring Harbor, NY.
Miller, M. J., and D. G.
Ahearn. 1987. Adherence
of Pseudomonas aeruginosa to hydrophilic contact lenses and other
substrata. J. Clin. Microbiol. 25(8):1392-1397.
Mittelman, M. W. (2001). “Microbially
Influenced Corrosion of Sprinkler Piping,” http://www.pmengineer.com/CDA/ArticleInformation/features/BNP__Features__Item/0,2732,24897,00.html.
Murray, J.D.,
Mathematical Biology, Springer-Verlag, 1993.
O'Toole, G. A., H. Kaplan, and
R. Kolter. 2000. Biofilm
formation as microbial development. Annu. Rev. Microbiol. 54:49-79.
O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility
are necessary for Pseudomonas aeruginosa biofilm development. Mol.
Microbiol. 30(2):295-304.
O'Toole, G. A., and R. Kolter. 1998. The initiation of biofilm
formation in Pseudomonas fluorescens WCS365 proceeds via multiple,
convergent signaling pathways: a genetic analysis. Mol. Microbiol. 28:449-461.
O'Toole, G. A., L. A. Pratt,
P. I. Watnick, D. K. Newman, V. B. Weaver, and R. Kolter. 1999. Genetic approaches to the study
of biofilms, p. 91-109. In R. J. Doyle (ed.), Methods in Enzymology,
vol. 310. Academic Press, San Diego, CA.
Pier, G. 1998. Pseudomonas aeruginosa: a
key problem in cystic fibrosis. ASM News. 64(6):339-347.
Pojman, J. A., Hyashenko, V.
M., Khan, A. M.,
"Free-radical frontal polymerization: self-propagating reaction
waves." J. Chem. Soc.,
Faraday Trans. 92, 2825 (1996).
Pratt, L. A., and R. Kolter. 1999. Genetic analysis of bacterial
biofilm formation. Curr. Opin. Microbiol. 2:598-603.
Pratt, L. A., and R. Kolter. 1998. Genetic analysis of Escherichia
coli biofilm formation: roles of flagella, motility, chemotaxis and type I
pili. Mol. Microbiol. 30(2):285-294.
Prigent-Combaret, C., O.
Vidal, C. Dorel, and P. Lejune.
1999. Abiotic surface sensing and biofilm-dependent regulation of gene
expression in Escherichia coli. J. Bacteriol. 181(19):5993-6002.
Sauer, K., and A. K. Camper. 2001. Characterization of phenotypic
changes in Pseudomonas putida in response to surface-associated growth.
J Bacteriol. 183(22):6579-89.
Stewart, P. S. 1998. A review of experimental
measurements of effective diffusive permeabilities and effective diffusion
coefficients in biofilms. Biotechnol Bioeng. 59(3):261-72.
Stewart, P. S. 1996. Theoretical aspects of
antibiotic diffusion into microbial biofilms. Antimicrob Agents Chemother. 40(11):2517-22.
Stover, C. K., X. Q. Pham, A.
L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O.
Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S.
Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K.
Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, and I. T. Paulsen. 2000. Complete genome sequence of
Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature.
406(6799):959-64.
Wichman, I. S. 1999. On Diffusion Flame Attachment
Near Cold Surfaces. Combustion and Flame 117:384-393.
Wichman, I. S., Olson, S. L. 1999. Investigation of Diffusion Flame
Tip Thermodiffusive and Hydrodynamic Instability Under Microgravity Conditions,
5th International Microgravity Combustion Workshop, Cleveland, OH,
May 1999.
Williams, F. A., Combustion Theory, 2nd Ed.,
Benjamin-Cummins, 1985
Winfree, A.T.,
The Geometry of Biological Time, Springer-Verlag, 1990.
Yassien M, Khardori N, Ahmedy
A, Toama M. (1995). Modulation of biofilms of Pseudomonas
aeruginosa by quinolones. Antimicrob Agents Chemother. 39:2262-8.